
If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say “monoxide” rather than “monooxide” and “trioxide” rather than “troxide”). Normally, no prefix is added to the first element’s name if there is only one atom of the first element in a molecule. Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. Chemical structure: This structure is also available as a 2d Mol fileor as a computed3d SD file. IUPAC Standard InChIKey:VZGDMQKNWNREIO-UHFFFAOYSA-NCopy.

A system of numerical prefixes is used to specify the number of atoms in a molecule. IUPAC Standard InChI:InChI1S/CCl4/c2-1(3,4)5Copy.
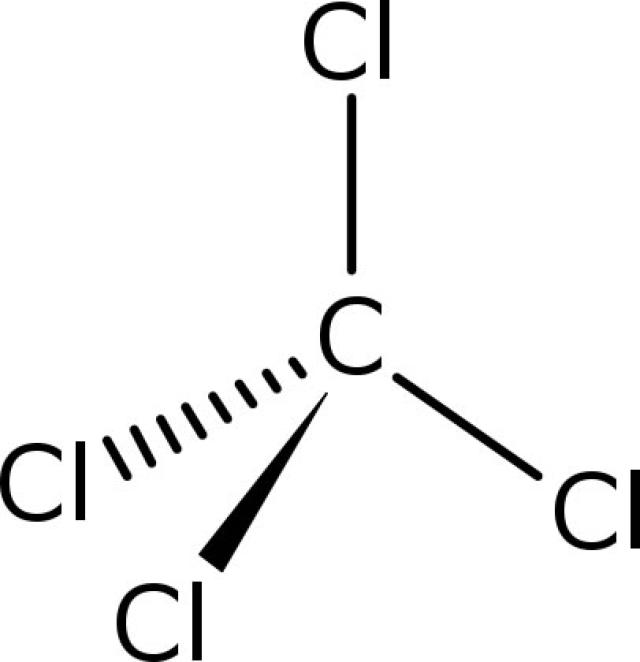
The second element is named by taking the stem of the element name and adding the suffix - ide. Classify each substance as an atomic element, molecular element, or. Carbon tetrachloride is a colourless liquid, used in fire extinguishers. Carbon tetrachloride or tetrachloromethane is an organic compound because it is composed of atoms from more than one element (in this example carbon and chlorine) held together by covalent chemical bonds. Predict whether the compound formed in each case is an ionic or a covalent compound. Answer is: carbon tetrachloride (CCl) is compound. The first element in the formula is simply listed using the name of the element. Classify each compound as ionic or molecular. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. For example, we have already seen CH 4, the molecular formula for methane. Numerical subscripts are used if there is more than one of a particular atom. Then the other nonmetal symbols are listed.

Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H 2O is the prominent exception). These compounds are very different from ionic compounds like sodium chloride (NaCl) ( NaCl). Examples include such familiar substances as water (H2O) ( H 2 O) and carbon dioxide (CO2) ( CO 2). Study with Quizlet and memorize flashcards containing terms like Carbon tetrachloride (CCl4 ) is used as a propellant in aerosol cans. because these compounds exist as separate, discrete molecules. Molecular compounds are inorganic compounds that take the form of discrete molecules. Thus, the compound formed from sodium and chlorine will be ionic (a. What is the name of the formula CBr4 First, you need to find out if this is a ionic or covalent compoundSince Carbon and Bromine are both non-metals, this is covalentName the first element. Compounds that involve a metal binding with either a non-metal will display ionic bonding. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Trending Questions Where was NASA's first headquarters? 0.The chemical formulas for covalent compounds are referred to as molecular formulas A chemical formula for a covalent compound. Covalent bonds form when two or more nonmetals combine.


 0 kommentar(er)
0 kommentar(er)
